Aqueous humour (fluid)
| Aqueous humour | |
|---|---|
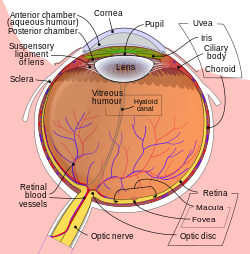 Schematic diagram of the human eye. | |
| Details | |
| Identifiers | |
| Latin | humor aquosus |
| MeSH | D001082 |
| TA98 | A15.2.06.002 |
| TA2 | 6791 |
| FMA | 58819 |
| Anatomical terminology | |
aqueous humour, optically clear, slightly alkaline liquid.
Your eyes continuously make aqueous humor,
It fills both the anterior and the posterior chambers of the eye, and is not to be confused with the vitreous humour, which is located in the space between the lens and the retina, also known as the posterior cavity or vitreous chamber.[2]
Blood cannot normally enter the eyeball.[3]
Structure[edit]
Composition[edit]
- Amino acids: transported by ciliary muscles
- 98% water
- Electrolytes (pH = 7.4 -one source gives 7.1[4])
- Ascorbic acid
- Glutathione
- Immunoglobulins
Function[edit]
- Maintains the intraocular pressure and inflates the globe of the eye. It is this hydrostatic pressure that keeps the eyeball in a roughly spherical shape and keeps the walls of the eyeball taut.
- Provides nutrition (e.g. amino acids and glucose) for the avascular ocular tissues; posterior cornea, trabecular meshwork, lens, and anterior vitreous.
- May serve to transport ascorbate in the anterior segment to act as an antioxidant agent.
- Presence of immunoglobulins indicates a role in immune response to defend against pathogens.
- Provides inflation for expansion of the cornea and thus increased protection against dust, wind, pollen grains, and some pathogens.
- For refractive index.
- Prevents eye dryness.
It provides these nutrients (as well as oxygen) to eye tissues that lack a direct blood supply (such as the lens) and also removes their waste products. In addition, it provides an internal pressure, known as intraocular pressure, that keeps the eyeball (globe) properly formed. Aqueous humour is formed from the blood by filtration, secretion, and diffusion through the ciliary body, a muscular structure located behind the iris that controls the curvature of the lens. Aqueous humour leaves the eye through the porous trabecular meshwork and flows into Schlemm’s canal, a ringlike passageway around the outer angle of the anterior chamber in front of the iris. From the canal the liquid enters the veins.
When the aqueous humour does not adequately drain from the eye, intraocular pressure can rise and loss of vision can result. Elevated eye pressures can contribute to the onset of many types of glaucoma, a common vision-threatening group of diseases. Therapies for glaucoma are aimed at lowering eye pressure by increasing the outflow of aqueous humour from the eye and decreasing its production by the ciliary body. Two types of surgery that increase outflow of fluid from the eye include trabeculoplasty, a type of laser surgery that increases the permeability of the trabecular meshwork, and trabeculectomy (also called filtering microsurgery). Trabeculectomy diverts aqueous humour from the anterior chamber inside the eye to the space under the conjunctiva (the transparent skin that covers the white area, or sclera, of the eye).
Imbalances in the creation and drainage of aqueous humor can lead to high intraocular pressure (eye pressure or IOP). High intraocular pressure is a major part of glaucoma and can damage vision.
Cellular Level
Active transport at the site of the non-pigmented epithelial cells is thought to be the major contributor to aqueous humor formation. The energy required for active transport is generated via the hydrolysis of ATP to ADP, mediated by the enzyme Na-K-ATPase, which is found on both pigmented and non-pigmented ciliary epithelia. Na-K-ATPase is of specific pharmacological interest as it can be inhibited by many different molecules, including cardiac glycosides, dinitrophenol, and acetazolamide. Carbonic anhydrase, an enzyme also found in pigmented and non-pigmented ciliary epithelia, mediates bicarbonate transport across ciliary epithelium via hydration of CO, forming HCO and protons.
The formation of bicarbonate regulates pH for optimal active ion transport, which influences fluid transport by affecting Na. Chloride (Cl) is the major anion transported across the epithelium through Cl channels. Other molecules actively transported include ascorbic acid and certain amino acids. Active transport produces an osmotic gradient across the ciliary epithelium, promoting the movement of other plasma components via ultrafiltration and diffusion.[2]
Aqueous humor also functions to remove waste products, blood, macrophages, and other debris from the posterior cornea and anterior lens and maintain the shape and IOP of the eyeball.
Production[edit]
Aqueous humour is secreted into the posterior chamber by the ciliary body, specifically the non-pigmented epithelium of the ciliary body (pars plicata). 5 alpha-dihydrocortisol, an enzyme inhibited by 5-alpha reductase inhibitors, may be involved in production of aqueous humour.[5]
Drainage[edit]
Aqueous humor is continually produced by the ciliary processes and this rate of production must be balanced by an equal rate of aqueous humor drainage. Small variations in the production or outflow of aqueous humor will have a large influence on the intraocular pressure.
The drainage route for aqueous humor flow is first through the posterior chamber, then the narrow space between the anterior iris and the posterior lens (contributes to small resistance), through the pupil to enter the anterior chamber. From there, the aqueous humor exits the eye through the trabecular meshwork into Schlemm's canal (a channel at the limbus, i.e., the joining point of the cornea and sclera, which encircles the cornea[6]) It flows through 25–30 collector canals into the episcleral veins. The greatest resistance to aqueous flow is provided by the trabecular meshwork (esp. the juxtacanalicular part), and this is where most of the aqueous outflow occurs. The internal wall of the canal is very delicate and allows the fluid to filter due to the high pressure of the fluid within the eye.[6] The secondary route is the uveoscleral drainage, and is independent of the intraocular pressure, the aqueous flows through here, but to a lesser extent than through the trabecular meshwork (approx. 10% of the total drainage whereas by trabecular meshwork 90% of the total drainage).
The fluid is normally 15 mmHg (0.6 inHg) above atmospheric pressure, so when a syringe is injected the fluid flows easily. If the fluid is leaking, the hardness of the normal eye is compromised, leading to collapse and wilting of the cornea.[6]
Clinical significance[edit]
Glaucoma is a progressive optic neuropathy where retinal ganglion cells and their axons die causing a corresponding visual field defect. An important risk factor is increased intraocular pressure (pressure within the eye) either through increased production or decreased outflow of aqueous humour.[7] Increased resistance to outflow of aqueous humour may occur due to an abnormal trabecular meshwork or due to obliteration of the meshwork resulting from injury or disease of the iris. However, increased interocular pressure is neither sufficient nor necessary for development of primary open angle glaucoma, although it is a major risk factor. Uncontrolled glaucoma typically leads to visual field loss and ultimately blindness.
Uveoscleral outflow of aqueous humour can be increased with prostaglandin agonists, while trabecular outflow is increased by M3 agonists. Fluid production can be decreased by beta blockers, alpha2-agonists, and carbonic anhydrase inhibitors.[8]
Aqueous Humor
Aqueous humor is the transparent fluid in the anterior and posterior chambers of the eye and is formed by the ciliary epithelium.
Control of Aqueous Humor Flow
J.W. McLaren, in Encyclopedia of the Eye, 2010
Aqueous humor circulation maintains the optical clarity of the cornea, anterior chamber, and crystalline lens, and is the source of intraocular pressure. Aqueous humor flows continuously and few conditions, other than sleep, affect its flow rate. Studies of the circulation of aqueous humor under a variety of conditions have provided an understanding of basic physiologic properties of the eye, as well as pharmacologic treatments for glaucoma. In this article, we will examine how aqueous humor flow rate is measured, what can change aqueous humor production, and how flow can be pharmacologically manipulated to treat glaucoma.
Toxocara and Toxocariasis
Paula Mayara Matos Fialho, ... Susana Zevallos Lescano, in Advances in Parasitology, 2020
4.2.1 TES-ELISA of the aqueous humour
Aqueous humour samples should be used for detecting anti-Toxocara antibodies in cases of OT. Diagnosis can be made by comparing the ELISA results in serum and aqueous humour samples, and applying the Goldmann-Witmer (GW) coefficient: (specific IgG level in aqueous humour/specific IgG level in serum)/(total IgG level in aqueous humour/total IgG in serum). The production of specific antibodies in the intraocular region is defined when the GW coefficient is higher than 3.0 (de Visser et al., 2008). However, ocular fluid examination is not a routine procedure in these cases, and laboratory diagnosis is performed using serum samples in clinical practice.
Few studies have correlated clinical data with serological test results and ophthalmic findings for diagnosing OT. Rubinsky-Elefant et al. (2018) analysed the correlation between the production of serum anti-Toxocara antibodies and the diagnosis of OT by evaluating common signs and symptoms in patients from a referral health centre in Brazil. The study has shown that OT affects children predominantly, and is characterised by visual loss, especially in the right eye. In addition, Toxocara larvae are located in the peripheral and posterior pole (Rubinsky-Elefant et al., 2018).
Ciliary Blood Flow and its Role for Aqueous Humor Formation
J.W. Kiel, H.A. Reitsamer, in Encyclopedia of the Eye, 2010
Introduction
Aqueous humor is the clear liquid in the posterior and anterior chambers of the eye. It is produced by the ciliary processes, flows through the posterior chamber, then between the iris and lens through the pupil into the anterior chamber, and then leaves the eye through the trabecular and uveoscleral outflow pathways. The flow of aqueous humor serves two vital functions – it is the surrogate vascular system for the cornea and lens, delivering nutrients and removing metabolic waste for these avascular tissues, and it generates the intraocular pressure (IOP), which maintains the shape of the eye and sets the venous pressure for the intraocular circulations.
Figure 1 shows a schematic overview of aqueous production. Aqueous humor is formed in the ciliary processes in the pars plicata region of the anterior uvea by the translocation of fluid and solute across the pigmented epithelial (PE) and nonpigmented epithelial (NPE) layers, the epithelial bilayer that comprises the primary blood–aqueous barrier. Aqueous production occurs in three steps: (1) convective delivery of the aqueous components and metabolic fuels through the ciliary circulation, (2) ultrafiltration and diffusion from the capillaries into the stroma driven by the oncotic pressure, hydrostatic pressure, and concentration gradients, and (3) ionic transport into the basolateral spaces between the NPE cells, followed by water movement down the resultant osmotic gradient into the posterior chamber. Once formed, the aqueous composition is modified as it travels through the posterior and anterior chambers by metabolic exchange with the tissues it contacts – the lens, iris, and cornea.
Aqueous humor, glaucoma, and corneal health
Carol B. Toris, ... Christine E. Martinez, in The Science of Glaucoma Management, 2023
22.3.2.3 Aqueous humor in diseases of the cornea
Aqueous humor composition alterations have been identified in various corneal diseases and states (Table 22.3), including corneal dystrophies, bullous keratopathy (Fig. 22.1), corneal transplants (Figs. 22.2–22.4), and corneal transplant rejection.
Table 22.3. Aqueous humor cytokines in corneal disease.
| Corneal disease | Elevated cytokines | References |
|---|---|---|
| PPCD | TGF-beta2 | Stadnikova et al. (2017) |
| CHED | Ascorbic acid | Guha et al. (2021) |
| Bullous keratopathy | IL-1-alpha, IL-4, MIP-1beta | Tomida et al. (2020) |
| Fuchs endothelial dystrophy and bullous keratopathy | IL-6, IL-8, GM-CSF, IFN-gamma, MCP-1, MIP-1beta | Fisenko et al. (2021) |
CHED, Congenital hereditary endothelial dystrophy; PPCD, posterior polymorphous corneal dystrophy.
Posterior polymorphous corneal dystrophy (PPCD) is a bilateral disease characterized by corneal endothelial cells whose features appear and behave like epithelial cells. These patients may develop secondary glaucoma, iris abnormalities, and corneal edema. Elevated TGF-beta2 levels are found in the aqueous humor of patients with PPCD compared to controls (Stadnikova et al., 2017). The significance of this finding is unclear, but a meta-analysis showed elevated levels of TGF-beta2 in aqueous humor of patients with open-angle glaucoma (Agarwal et al., 2015). Another corneal dystrophy, congenital hereditary endothelial dystrophy (CHED) is a disease of endothelial dysfunction that leads to corneal edema in infants and children. Elevated levels of ascorbic acid were found in the aqueous humor of eyes with CHED compared to control eyes with congenital cataracts (Guha et al., 2021). The significance of this finding is unclear.
Healthy corneal endothelial and epithelial cell layers serve barrier functions and in bullous keratopathy, these barrier functions are compromised. Elevated levels of tear protein, aqueous humor protein, and aqueous humor cytokines (IL-1-alpha, IL-4, MIP-1beta) were reported in patients with bullous keratopathy compared to controls. Additionally, tear cytokine levels correlated with aqueous humor cytokine levels in eyes with bullous keratopathy, but not in eyes with corneal scars, ectasia, stromal dystrophies, or healthy corneas. These findings indicate that loss of barrier function in bullous keratopathy is associated with altered aqueous humor composition and potentially reflects increased communication between the tear film, cornea, and aqueous humor (Tomida et al., 2020). Additionally, elevated levels of inflammatory cytokines (interleukin-6, interleukin-8, and granulocyte macrophage colony stimulating factor; interferon-gamma; monocyte chemotactic protein-1; macrophage inflammatory protein-1beta) were found in both Fuchs endothelial dystrophy and bullous keratopathy compared to control eyes (Fisenko et al., 2021) (Fig. 22.1; Table 22.3).
In patients undergoing either Descemet stripping automated endothelial keratoplasty or penetrating keratoplasty, elevated preoperative levels of various aqueous humor cytokines are associated with higher rates of postoperative endothelial cell loss (Yagi-Yaguchi et al., 2017; Yazu et al., 2018). In one recent study, the aqueous humor levels of severe proinflammatory cytokines were lower in eyes that had undergone Descemet membrane endothelial keratoplasty compared to eyes with bullous keratopathy that had not yet undergone corneal transplantation (Hayashi et al., 2021). This suggests that restoration of healthy endothelial pump function plays a role in regulation of aqueous humor composition. These findings underscore the relationship between aqueous humor composition and corneal disease in corneal transplant patients (Figs. 22.2–22.4).
NK cells in the eye
Jerry Y. Niederkorn, in Natural Killer Cells, 2010
Anti-Inflammatory and Immunosuppressive Soluble Factors in the Eye
The aqueous humor (AH) that fills the AC of the eye contains a myriad of anti-inflammatory and immunosuppressive molecules (Taylor, 2007). At least four different AH-borne factors inhibit the expression of T cell–mediated inflammation such as delayed-type hypersensitivity (DTH): (a) transforming growth factor-β (TGF-β), (b) α-melanocyte stimulating hormone (α-MSH), (c) vasoactive intestinal peptide (VIP) and (d) calcitonin gene-related peptide (CGRP) (Cousins et al., 1991; Granstein et al., 1990; Taylor, 2007; Taylor and Yee, 2003; Taylor et al., 1994a,b). The AH also contains a 10 KDa peptide that induces apoptosis of NK cells, T cells, macrophages and neutrophils (D’Orazio et al., 1999). Corneal cells also produce indoleamine dioxygenase (IDO), an enzyme that catabolizes tryptophan, which is a key amino acid that is vital for T cell survival (Beutelspacher et al., 2006; Ryu and Kim, 2007) and the proliferative and cytolytic activity of NK cells (Della Chiesa et al., 2006; Frumento et al., 2002). Thus, cells of both the adaptive and innate systems entering the eye are greeted by factors that inhibit their function and survival.
The AH contains a variety of molecules that suppress the innate immune system. A 10 KDa factor induces apoptosis of NK cells, macrophages and neutrophils. In addition to cellular elements, the innate immune system employs soluble factors such as the complement system. The classical pathway of the complement cascade can be activated when complement-fixing antibodies bind to their cognate antigens or by the alternative pathway via interactions with microorganisms. Thus, the complement system straddles the innate and adaptive immune systems. Once activated, the complement cascade generates a variety of potent chemoattractants that recruit and activate granulocytes. Granulocytes produce a wide array of toxic reactive oxygen species and proteases that can inflict irreparable injury to ocular cells. However, the untoward effects of complement activation are prevented by complement regulatory proteins (CRP) that are present in the AH and vitreous body and that inactivate the complement cascade before it can cause harm (Goslings et al., 1998; Lass et al., 1990; Sohn et al., 2000a,b).
The AH also contains several soluble factors that affect the function of NK cells. Macrophage migration inhibitory factor (MIF) and TGF-β are present in the AH at concentrations that inhibit NK cell cytolytic activity (Apte and Niederkorn, 1996; Apte et al., 1997, 1998; Rook et al., 1986). Although MIF is generally associated with inflammation, it has remarkable inhibitory effects on the cytolytic activity of NK cells. MIF inhibits the release of perforin molecules by NK cells but not CTL (Apte et al., 1998). MIF acts almost immediately and inhibits NK cell-mediated cytolysis of NK-sensitive tumour cells within 4 h. By contrast, TGF-β-mediated inhibition of NK cytolytic activity is not detected until 18 h (Rook et al., 1986). NK cells lyse corneal endothelial cells in vitro, due to their absence of MHC class I molecules that provide an “off” signal to NK cells (Apte and Niederkorn, 1996; Apte et al., 1998). However, this cytolysis is inhibited if recombinant MIF or AH is added to the cytolysis assay system, suggesting that in the AC of the eye, MIF and TGF-β inhibit NK cellular cytotoxicity (Apte and Niederkorn, 1996). In support of this are studies showing that syngeneic tumours that undergo NK cell-mediated rejection at extraocular sites (e.g. subcutaneous), grow progressively in the AC of the eye (Apte et al., 1997).
The Eye and Vision
R.A. Armstrong, R.P. Cubbidge, in Handbook of Nutrition, Diet and the Eye, 2014
Aqueous Humor
The aqueous humor is a transparent liquid produced by the ciliary body and which passes through the pupil, thus filling the anterior chamber. It ultimately passes through a fine meshwork at the cornea–sclera–iris junction called the trabecular meshwork into Schlemm canal, and then drains into the venous system. Aqueous humor has a consistency much like water and functions to provide nutrients to, and remove waste products of, metabolism from the transparent cornea and crystalline lens, both of which do not possess a blood supply. The continuous production of the aqueous humor and drainage through the trabecular meshwork results in a fluid pressure that has a range in normal individuals of 10–20 mm Hg. This pressure serves to maintain the shape of the eye. In some individuals, the trabecular meshwork can suddenly or more gradually become blocked leading to an increase in intraocular pressure (IOP) in the anterior chamber. This increase in IOP is transferred to the retina damaging its function, leading to a condition called glaucoma.8
Diseases of the eye
Anastasia P. Nesterova, ... Anton Yuryev, in Disease Pathways, 2020
Key cellular contributors and processes
Process
Aqueous humor (AH) is a transparent liquid that occupies the space between the crystalline lens and the cornea of the eye. AH resembles plasma, but it contains lower protein and glucose concentrations. AH nourishes the cornea and the lens and is involved in intraocular pressure maintenance.
Aqueous humor production and function
Process
Regulation of aqueous humor (AH) outflow is important for intraocular pressure maintenance. The drainage path for AH starts in the posterior chamber of the eye. Further, AH flows into the area between the posterior iris and the anterior lens and then through the pupil and enters the anterior chamber. From the anterior chamber, AH leaves the eye through the trabecular meshwork (TM) and flows into Schlemm’s canal. Further, AH flows through the collector channels into the episcleral veins. The greatest resistance to AH outflow is contained at the TM.
Process
Intraocular pressure (IOP) is the intraocular fluid pressure inside the eyeball. IOP depends on the balance between the production and drainage of aqueous humor mainly through the trabecular meshwork. IOP is increased in glaucoma.
Optic nerve
The optic nerve (cranial nerve II, CN II) is a paired nerve that conducts visual impulses from the retina to the brain. The optic nerve consists of retinal ganglion cell axons and glial cells. In glaucoma, the optic nerve is damaged due to increased intraocular pressure.
Anatomic structure
The trabecular meshwork (TM) is an area in the anterior chamber of the eye lined by cells called trabeculocytes. The TM provides resistance to aqueous humor flow and is crucial for intraocular pressure maintenance.
Aqueous humor formation
Robert L Stamper MD, ... Michael V Drake MD, in Becker-Shaffer's Diagnosis and Therapy of the Glaucomas (Eighth Edition), 2009
FUNCTION OF AQUEOUS HUMOR
Aqueous humor was originally thought to be stagnant. It was not until 1921 that Seidel proved that the aqueous was, indeed, circulating. Using a needle, Seidel connected a reservoir containing a blue dye to a rabbit eye. When the reservoir was lowered, clear fluid from the anterior chamber entered the tubing; when the reservoir was raised, the dye entered the eye and eventually appeared in the blood of the episcleral venous plexus.1,2 Seidel concluded that aqueous humor must be continuously formed and drained. Two decades later, Ascher showed that aqueous humor enters the venous system at the limbus through the aqueous veins and first flows alongside the bloodstream in a laminar fashion before mixing completely with the blood in the veins.3 Ashton studied neoprene casts of Schlemm's canal and the aqueous veins and demonstrated a direct connection between these two structures.4 From the work of the last half century it is clear that aqueous humor is a relatively cell-free, protein-free fluid that is formed by the ciliary body epithelium in the posterior chamber. It then passes between the iris and the lens, enters the anterior chamber through the pupil, and exits the eye at the anterior chamber angle through the trabecular meshwork, Schlemm's canal, and the aqueous veins. In the anterior chamber, the aqueous humor is subject to thermal currents because of the temperature difference between the iris and the cornea; aqueous rises close to the warmer iris and descends close to the cooler cornea. This convection current may easily be seen clinically when there are cells or pigment in the anterior chamber, and explains the relatively inferior location of pigment deposition (Krukenberg spindle) and keratic precipitates on the inner surface of the cornea.
During its passage through the eye, the aqueous humor serves a number of important functions. It serves in lieu of a vascular system for the normally avascular structures of the eye, including the cornea, lens, and trabecular meshwork. It brings to the internal eye essential nutrients, such as oxygen, glucose, and amino acids,5 and removes metabolites and potentially toxic substances, such as lactic acid and carbon dioxide.6,7 Aqueous humor provides the proper chemical environment for the tissues of the anterior segment of the eye and provides an optically clear medium to allow good visual function. It inflates the globe and maintains intraocular pressure (IOP), both of which are important for the structural and optical integrity of the eye. In many species, including humans, aqueous humor contains a very high concentration of ascorbate, which may act to scavenge free radicals and protect the eye against the effects of ultraviolet and other radiation. Under adverse conditions (e.g., inflammation, infection), it facilitates cellular and humoral immune responses. During inflammation, the rate of aqueous humor formation decreases, and its composition is altered to permit accumulation of immune mediators (Box 2-1).
Several risk factors probably contribute to damaging the optic nerve with its resultant visual loss in glaucoma. Intraocular pressure that is too high for the continued health of the nerve is universally accepted as one of the most important of those risk factors. Therefore the study of those elements that contribute to the creation, maintenance, and variation of IOP is material to the understanding of the pathophysiology of this disease. Aqueous formation (F), facility of outflow (C), and episcleral venous pressure (Pv) are the major intraocular determinants of IOP. These factors are related to one another by the Goldmann equation:
or if solving for F:
in which PO is the IOP in the undisturbed eye in mmHg, aqueous formation is in μl/min, the facility of outflow is in μl/min/mmHg, and the episcleral venous pressure is in mmHg. From the equation, it is evident that IOP will increase when the aqueous formation rate increases, the episcleral venous pressure increases, or the outflow facility decreases. More recently, with the discovery of a pressure-independent outflow mechanism(s) (the uveoscleral pathway being the main one), the equation has had to be modified and is better stated:
where Pe is the sum of the external pressure such as episcleral venous pressure and other tissue pressures outside the eye, and U is the sum of the pressure-independent outflow pathways.8
Glaucoma and Antioxidant Status
Mehmet Tosun, ... Mesut Erdurmuş, in Handbook of Nutrition, Diet and the Eye, 2014
Oxidative Stress in the Pathophysiology of Glaucoma
Aqueous humor is an important fluid in the physiology of the human eye. It is secreted by the ciliary epithelium at a flow rate of 2–3 μL/minute, enters the posterior chamber, passes through the pupil into the anterior chamber, and then to the trabecular meshwork (TM) in the anterior chamber angle. It provides nutrients to anterior and posterior chamber structures and removes metabolic wastes. Aqueous humor also helps to maintain IOP in physiologic ranges. The TM, which drains the aqueous humor into the Schlemm canal, is composed of collagen fibers and elastic tissue covered by trabecular cells. These cells have phagocytic activity and may produce matrix metalloproteinase enzymes, extracellular matrix components, and certain growth factors.10–12 The mechanism of elevated IOP in open-angle glaucoma is impaired outflow of aqueous humor resulting from increased resistance within the TM, particularly in the juxtacanalicular portion.
Oxidative stress may have a role in the pathogenesis of glaucoma, through either TM degeneration or RGC loss. TM degeneration by oxidative stress has been implicated as causing increased IOP, thus contributing to alterations in the aqueous outflow pathway.13 The progressive loss of TM cells in patients with glaucoma may be ascribed to the long-term oxidative damage induced by ROS.14,15 Treatment of human TM cells in vitro with hydrogen peroxide impairs TM cell adhesion and compromises cytoskeletal cell integrity. It has also been demonstrated that oxidative DNA damage is significantly greater in the TM cells of glaucoma patients, compared with controls.16 Furthermore, in vivo studies have demonstrated that both IOP increase and visual field damage are related to the amount of oxidative DNA damage.12,17
The endothelium may be involved in the modulation of vascular permeability by the release of endothelins and nitric oxide (NO), which are important oxidants that can worsen TM metabolism.18 Endothelins may also influence TM motility and IOP regulation.19 Aqueous humor endothelin levels have been demonstrated to be higher in glaucoma patients than in unaffected controls.20 As a result, various mechanisms can contribute to the production of oxidizing free radicals in the TM. However, a predominant role is played by the endogenous aerobic metabolism, which may be abetted by vascular dysregulation.21
Increasing evidence supports the idea that the pathogenic mechanism leading to glaucomatous RGC loss is oxidative stress. Oxidative stress induces apoptotic cell death by the activation of c-Jun N-terminal kinase (stress-activated protein kinase) and p38 mitogen-activated protein kinase, leading to caspase 3 activation.22 It is hypothesized that fluctuations of ocular blood flow in patients with normal IOP can lead to ischemia reperfusion injury and result in oxidative tissue damage.23 This interpretation is in agreement with the vascular pathogenic theory that suggests vessel dysregulation as the fundamental pathogenetic step in POAG.24 Peripheral vascular insufficiency and restoration of blood flow induces a proinflammatory state reflected by enhanced O2- and H2O2 generation, which places organs at risk. These ROSs are usually derived from mitochondrial autoxidation, and they are also found within the RGCs, nerve fiber layer, outer plexiform layer, inner segments of photoreceptors, and the retinal pigment epithelium.25 Increased ROS generation leads to RGC degeneration, glial malfunction, and activation of autoimmune response in glaucoma. However, many aspects of the relationship between oxidative stress and the neurodegenerative course remain obscure. During glaucomatous neuronal cell degeneration, ROS may be directly neurotoxic to RGCs, may cause secondary degeneration by stimulating glial dysfunction, and may also function as a second messenger and/or in regulating redox modifications of downstream effectors.6
PEX syndrome is characterized by the production and progressive deposition of fibrilogranular extracellular material in ocular tissues; it is most commonly seen on the pupillary border and anterior lens capsule.26 Accumulation of the PEX material or pigment particles in the anterior chamber angle can predispose the individual to both open-angle and angle-closure glaucoma.12,27 Reported findings also suggest a role for oxidative stress in the pathogenesis and progression of PEX glaucoma.
Table 9.1 shows the current evidence implicating oxidative stress in the pathophysiology of glaucoma.
TABLE 9.1. Current Evidence Implicating Oxidative Stress in the Pathophysiology of Glaucoma
| Tissue | Oxidative Parameter∗ | References |
|---|---|---|
| Aqueous humor | Antioxidant status/enzyme activity | 3,7,20,28–31 |
| DNA damage | 31 | |
| Trabecular meshwork | Antioxidant status/enzyme activity | 4,18,32 |
| DNA damage | 4,17 | |
| Retina/vitreous | Antioxidant status/enzyme activity | 49 |
| Serum | Antioxidant status/enzyme activity | 29,31,33–38 |
| DNA damage | 31,35,38 |
DNA: deoxyribonucleic acid.
- ∗
- These studies represent investigations on human subjects.
Cannabinoid Regulation of Intraocular Pressure: Human and Animal Studies, Cellular and Molecular Targets
A. Aloway, ... Z.H. Song, in Handbook of Cannabis and Related Pathologies, 2017
Ciliary Epithelium
Aqueous humor is produced and secreted by the ciliary epithelium. Therefore, interactions between cannabinoids and the ciliary epithelium is a mechanism by which IOP can be controlled. Ciliary epithelium expresses high levels of CB1, as shown by immunohistochemistry (Straiker et al., 1999).
Morphological changes in the ciliary epithelium have been observed after exposure to marijuana. Specifically, monkey ciliary epithelium experienced swelling, followed by a reduction in IOP after intravitreal injection of soluble marijuana extracts (McDonald, Cheeks, Slagle, & Green, 1991).
Further mechanistic studies demonstrated that cannabinoid-induced IOP reduction may involve prostaglandins and metalloproteinase produced by nonpigmented ciliary epithelium. Treatment of human nonpigmented ciliary epithelium with R(+)-methanandamide, a CB1 agonist, resulted an increase in cyclooxygenase-2 (COX-2) mRNA expression. Increased COX-2 expression was followed by increased phosphorylation of p38 and p42/44 mitogen-activated protein kinase (MAPK), two key signaling molecules in metalloproteinase (MMP) regulation. Correspondingly, mRNA and protein expression of MMP-1, MMP-3, MMP-9, and tissue inhibitor of metalloproteinase 1 (TIMP-1) was increased; therefore a potential mechanism contributing to the IOP-lowering effects of cannabinoids may be the stimulation of COX-2 and MMP expression in nonpigmented ciliary epithelium (Rosch, Ramer, Brune, & Hinz, 2006).





Post a Comment