Sodium distribution in species[edit]
Humans[edit]
The minimum physiological requirement for sodium is between 115 and 500 mg per day depending on sweating due to physical activity, and whether the person is adapted to the climate.[5] Sodium chloride is the principal source of sodium in the diet, and is used as seasoning and preservative, such as for pickling and jerky; most of it comes from processed foods.[6] The Adequate Intake for sodium is 1.2 to 1.5 g per day,[7] but on average people in the United States consume 3.4 g per day,[8][9] the minimum amount that promotes hypertension.[10] Note that salt contains about 39.3% sodium by mass[11]—the rest being chlorine and other trace chemicals; thus the Tolerable Upper Intake Level of 2.3 g sodium would be about 5.9 g of salt—about 1 teaspoon.[12] The average daily excretion of sodium is between 40 and 220 mEq.[13]
Normal serum sodium levels are between approximately 135 and 145 mEq/L (135 to 145 mmol/L). A serum sodium level of less than 135 mEq/L qualifies as hyponatremia, which is considered severe when the serum sodium level is below 125 mEq/L.[14][15]
The renin–angiotensin system and the atrial natriuretic peptide indirectly regulate the amount of signal transduction in the human central nervous system, which depends on sodium ion motion across the nerve cell membrane, in all nerves. Sodium is thus important in neuron function and osmoregulation between cells and the extracellular fluid; the distribution of sodium ions are mediated in all animals by sodium–potassium pumps, which are active transporter solute pumps, pumping ions against the gradient, and sodium-potassium channels.[16] Sodium channels are known to be less selective in comparison to potassium channels. Sodium is the most prominent cation in extracellular fluid: in the 15 L of extracellular fluid in a 70 kg human there is around 50 grams of sodium, 90% of the body's total sodium content.
Some potent neurotoxins, such as batrachotoxin, increase the sodium ion permeability of the cell membranes in nerves and muscles, causing a massive and irreversible depolarization of the membranes with potentially fatal consequences. However, drugs with smaller effects on sodium ion motion in nerves may have diverse pharmacological effects that range from anti-depressant to anti-seizure actions.
Urinary sodium[edit]
Because the hypothalamus/osmoreceptor system ordinarily works well to cause drinking or urination to restore the body's sodium concentrations to normal, this system can be used in medical treatment to regulate the body's total fluid content, by first controlling the body's sodium content. Thus, when a powerful diuretic drug is given which causes the kidneys to excrete sodium, the effect is accompanied by an excretion of body water (water loss accompanies sodium loss). This happens because the kidney is unable to efficiently retain water while excreting large amounts of sodium. In addition, after sodium excretion, the osmoreceptor system may sense lowered sodium concentration in the blood and then direct compensatory urinary water loss in order to correct the hyponatremic (low blood sodium) state.
Sodium-glucose symporter[edit]

In the sodium-glucose symporter, sodium moves down it's concentration gradient to move glucose up it's concentration gradient. Sodium has a greater concentration outside of the cell, and binds to the symporter, which is in its outward facing confirmation. Once sodium is bound, glucose can bind from the extracellular space, causing the symporter to switch into the occluded formation (closed) before opening to the inside of the cell and releasing the 2 sodium ions and the 1 glucose molecule. Once both are released, the symporter re-orients itself to the outward facing conformation and the process starts all over again.[4] A major example of up- regulation of the sodium-glucose symporter is seen in patients with type-2 diabetes, where there is roughly a 3-4 fold up-regulation of the sodium-glucose symporter (SGLT1). This leads to an influx of glucose into the cell and results in hyperglycemia.[27]
Sodiums role in the Cystic Fibrosis Transport Regulator (CFTR)[edit]
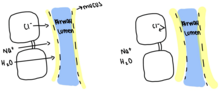
The Cystic Fibrosis Transport Regulator (CFTR) works by binding 2 ATP to the A1 and A2, ATP-binding domain. This opens the CFTR channel and allows chloride ions to flow into the lungs and airway lumen. This influx of negatively charged chloride ions into the airway lumen causes sodium to move into the airway lumen to balance the negative charge. Water then moves in with the sodium to balance the osmotic pressure and ultimately leads to the thinning of mucus. In cases of Cystic Fibrosis, the CFTR is defective and only binds a singular ATP, leading to the channel failing to open and preventing chloride ions from diffusing into the airway lumen. Since chloride ions cannot diffuse in, there is no movement of sodium into the airway lumen, and no need for water to move into the lumen, leading to thick mucus that clogs and infects the airway lumen.[4]\
SGLT2 inhibitors for diabetes[edit]
SGLT2 inhibitors, also called gliflozins,[5] are used in the treatment of type 2 diabetes. SGLT2 is only found in kidney tubules and in conjunction with SGLT1 resorbs glucose into the blood from the forming urine. By inhibiting SGLT2, and not targeting SGLT1, glucose is excreted which in turn lowers blood glucose levels. Examples include dapagliflozin (Farxiga in US, Forxiga in EU), canagliflozin (Invokana) and empagliflozin (Jardiance). Certain SGLT2 inhibitors have shown to reduce mortality in type 2 diabetes.[6] The safety and efficacy of SGLT2 inhibitors have not been established in patients with type 1 diabetes, and FDA has not approved them for use in these patients.[7]
إرسال تعليق