CSF is derived from blood plasma and is largely similar to it, except that CSF is nearly protein-free compared with plasma and has some different electrolyte levels. Due to the way it is produced, CSF has a higher chloride level than plasma, and an equivalent sodium level.[2][5]
What does cerebrospinal fluid consist of?
The electrolytes found in cerebrospinal fluid are sodium (Na+), chloride (Cl-), glucose, potassium (K+), calcium (Ca++), magnesium (Mg+), and . These are needed for osmoregulation, transport, neurotransmitter function, and conduction within the brain and spine. There are also other compounds present like enzymes, neurotransmitters and .
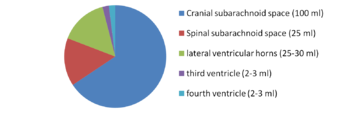
Summary The cerebrospinal fluid (CSF) is contained in the brain ventricles and the cranial and spinal subarachnoid spaces. The mean CSF volume is 150 ml, with 25 ml in the ventricles and 125 ml in subarachnoid spaces. CSF is predominantly, but not exclusively, secreted by the choroid plexuses. Brain interstitial fluid, ependyma and capillaries may also play a poorly defined role in CSF secretion. CSF circulation from sites of secretion to sites of absorption largely depends on the arterial pulse wave. Additional factors such as respiratory waves, the subject’s posture, jugular venous pressure and physical effort also modulate CSF flow dynamics and pressure. Cranial and spinal arachnoid villi have been considered for a long time to be the predominant sites of CSF absorption into the venous outflow system. Experimental data suggest that cranial and spinal nerve sheaths, the cribriform plate and the adventitia of cerebral arteries constitute substantial pathways of CSF drainage into the lymphatic outflow system. CSF is renewed about four times every 24 hours. Reduction of the CSF turnover rate during ageing leads to accumulation of catabolites in the brain and CSF that are also observed in certain neurodegenerative diseases. The CSF space is a dynamic pressure system. CSF pressure determines intracranial pressure with physiological values ranging between 3 and 4 mmHg before the age of one year, and between 10 and 15 mmHg in adults. Apart from its function of hydromechanical protection of the central nervous system, CSF also plays a prominent role in brain development and regulation of brain interstitial fluid homeostasis, which influences neuronal functioning. © 2011 Elsevier Masson SAS. All rights reserved
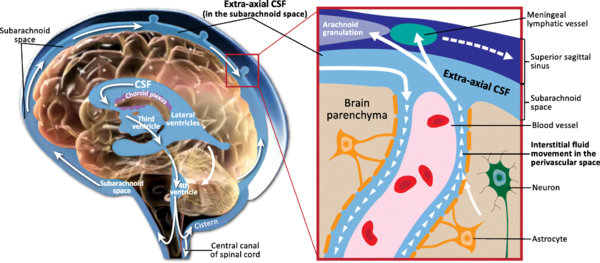
For a long time, the essential function of cerebrospinal fluid (CSF) was considered to be that of a fluid envelope that protects the central nervous system. Recent data derived from molecular biology show that CSF plays an essential role in homeostasis of the interstitial fluid of the brain parenchyma and regulation of neuronal functioning. Disorders of CSF hydrodynamics and composition are responsible for the major alterations of cerebral physiology observed in hydrocephalus and dementia, reflecting the importance of exchanges between CSF and the neuronal environment. Comparative anatomy Comparative anatomy of the meninges helps to elucidate the functional anatomy and ontogenesis of the CSF system in man [1]. The appearance of cerebrospinal fluid inside the neuraxis precedes circulation of cerebrospinal fluid in subarachnoid spaces during phylogenesis [2] The single primitive meninx with a large venous sinus in the spinal perimeningeal tissue of Selachii suggests the presence of a CSF venous absorption system. Large Teleostei present a pial layer lined by reticular tissue prefiguring the arachnoid membrane, but with no real CSF spaces. CSF is therefore contained in ventricular cavities. A peripheral fibrous layer differentiates and the perimeningeal tissue develops into an adipose tissue, which prefigures spinal epidural fat. In amphibians, reptiles and birds, the meninges comprise a dura mater and a pia mater. The perimeningeal tissue is considerably reduced, persisting at the spinal level in the form of epidural fat. In mammals, the subarachnoid space is clearly distinct from the pia mater. Participation of the central nervous system venous drainage in CSF absorption is first observed in Amniotes and is enhanced in the course of phylogenesis. Intracranial venous sinuses derived from cerebral epidural veins, and subarachnoid spaces develop in parallel [2]. Spinal epidural veins regress with a smaller participation in CSF absorption. The development of cerebrospinal fluid spaces retraces the steps of phylogenesis Cerebral and spinal meninges are derived from different embryonic tissues The three meningeal layers differentiate at the third month of intrauterine life. The meninges play a role in ontogenesis of the underlying brain tissue by inducing proliferation and differentiation of neuroblasts and axonal growth [3]. Experimental destruction of fetal meninges over the cerebellum induces cerebellar hypoplasia, neuronal ectopia and the formation of glial tissue in subarachnoid spaces [4,5]. Certain multiple malformation syndromes, such as Dandy Walker syndrome, comprising hypoplasia of the vermis and abnormalities of the cerebellar parenchyma and CSF spaces, could be due to similar mechanisms. The formation of subarachnoid spaces is not exclusively due to cerebrospinal fluid pressure On closure of the rostral and caudal neuropores at the first month of intrauterine life, the choroid plexuses are not yet functional [6]. However, CSF pressure increases in the lumen of the neural tube and the volume of the cephalic extremity increases, suggesting secretion of CSF by structures other than the choroid plexuses. The subarachnoid spaces appear on the 32nd day at the ventral aspect of the rhombencephalon, then extend caudally and dorsally However, the fourth ventricle is not yet open and CSF circulation is only effective on the 41st day. Formation of the subarachnoid spaces is therefore not exclusively due to CSF pressure. Formation of the subarachnoid spaces remains poorly understood. Capillaries appear to play a decisive role in the secretion and absorption of CSF during embryogenesis. Arachnoid cysts, dilatations of subarachnoid spaces predominantly located around blood vessels, appear to correspond to CSF spaces partly communicating with adjacent circulating blood sinuses. The first choroid plexuses The first choroid plexuses appear on the 41st day in the 4th ventricle [8]. The epithelium of the choroid plexus, continuous with the ependyma, is derived from the neural tube, while the leptomeningeal axis is derived from the paraxial mesoderm. The time at which the choroid plexuses start to secrete CSF has not been clearly determined. Arachnoid villi develop from the wall of intracranial venous sinuses From the 26th week, cerebral veins dilate at their anastomosis in the superior sagittal sinus. Villi are formed at the 35th week: the arachnoid stroma lined by endothelium protrudes into the lumen of the superior sagittal sinus via a defect in the dura mater. Real arachnoid granulations appear at the 39th week [9] and continue to develop until the age of about 18 months [10,11]. Cranial arachnoid granulations are essentially situated in contact with the posterior half of the superior sagittal sinus and adjacent venous lacunae and more rarely in contact with the transverse, superior petrosal, cavernous and sphenoparietal sinuses. These granulations ensure the bulk of CSF absorption at the end of organogenesis. However, comparative anatomy suggests other sites of CSF absorption in the absence of arachnoid villi or granulations. Volumes The CSF volume, estimated to be about 150 ml in adults, is distributed between 125 ml in cranial and spinal subarachnoid spaces and 25 ml in the ventricles, but with marked interindividual variations. Abnormally narrow ventricles, described as ‘‘slit ventricles’’, are observed in complex disorders of CSF circulation associated with cerebral oedema in patients with a CSF shunt. Inversely, hydrocephalus corresponds to an increased intracranial fluid volume and can be difficult to distinguish from cerebral atrophy, in which passive expansion of CSF spaces compensates for the reduction of brain volume. The distribution of fluid overload depends on the site of obstruction. In obstructive hydrocephalus, the obstruction is situated in the ventricular system, while in communicating hydrocephalus, the ventricular system and subarachnoid spaces freely communicate. The mechanisms of ventricular dilatation remain hypothetical, but include hydrodynamic factors (secretion and absorption rates, fluid pressure and cerebral compliance), hormonal neuropeptides, Atrial Natriuretic Peptide (ANP), and prostaglandin F2 (PGF2) [12]. Cerebrospinal fluid secretion Cerebrospinal fluid secretion in adults CSF secretion in adults varies between 400 to 600 ml per day, depending on the subject and the method used to study CSF secretion. Sixty to seventy-five percent of CSF is produced by the choroid plexuses of the lateral ventricles and the tela choroidea of the third and fourth ventricles. The choroid plexuses consist of granular meningeal protrusions into the ventricular lumen, the epithelial surface of which is continuous with the ependyma. They comprise a tuft of fenestrated capillaries. Choroidal cells present microvilli at their apical pole and are interconnected by tight junctions with a variable distribution according to the site on the ventricular wall [13]. Choroidal secretion of cerebrospinal fluid comprises two steps The first step consists of passive filtration of plasma from choroidal capillaries to the choroidal interstitial compartment according to a pressure gradient. The second step consists of active transport from the interstitial compartment to the ventricular lumen across the choroidal epithelium, involving carbonic anhydrase and membrane ion carrier proteins. Cytoplasmic carbonic anhydrase catalyses the formation of H+ and HCO3 − ions from water and CO2. The carrier proteins of basolateral membranes of choroidal cells exchange H+ and HCO3 − ions for Na+ and Cl− ions. ATP-dependent ion pumps of the apical membrane expel Na+, Cl−, HCO3 − and K+ ions towards the ventricular lumen. Water transport, facilitated by aquaporins I of the apical membrane, follows the osmotic gradients generated by these pumps [14]. The NaK2Cl cotransporter of the apical membrane generates ion transport in both directions and participates in regulation of CSF secretion and composition. Choroid plexuses secrete growth factors that probably act on the subventricular zone, which could repair tissue changes related to hydrocephalus, for example. They secrete vitamins B1, B12, C, folate, 2-microglobulin, arginine vasopressin and NO. Twenty percent of the peptides of CSF are derived from the brain and their concentration decreases as CSF flows from the ventricles to the subarachnoid spaces [15]. Extrachoroidal secretion Extrachoroidal secretion is derived from extracellular fluid and cerebral capillaries across the blood-brain barrier. This pathway appears to play a minimal role under physiological conditions. CSF can also be derived from the ependymal epithelium, the target of regulations mediated by neuropeptides and growth factors, which can be altered by ependymal changes induced, in particular, by ventricular dilatation. The composition of cerebrospinal fluid is not simply a plasma ultrafiltrate Na, Cl and Mg concentrations are higher and K and Ca concentrations are lower than those of plasma. The CSF cell count usually does not exceed five cells per milliliter. Variations in the closely regulated composition of CSF can be used for diagnostic purposes. Studies have demonstrated the existence of chronobiological cycles of Na content with peaks Na concentrations at 8:00 a.m. and at 6:00 p.m., with no modification of K and osmolarity. A relationship between the Na concentration and migraine has been proposed, as these peaks appear to correspond to the timing of migraine attacks [16]. Cerebrospinal fluid secretion and composition are finely regulated An increase in intraventricular pressure decreases the pressure gradient across the blood-brain barrier and decreases plasma filtration, but the capacities of adaptation of CSF secretion to intraventricular pressure at the initiation phase of hydrocephalus are rapidly exceeded. The choroid plexuses receive cholinergic, adrenergic, serotoninergic and peptidergic autonomic innervation. The sympathetic nervous system reduces CSF secretion, while the cholinergic system increases CSF secretion. The autonomic nervous system could be responsible for circadian variations of CSF secretion. Enzymes and membrane transporters are the targets of humoral regulation. Acid-base disorders modify the activity of carbonic anhydrase, aquaporins and membrane carrier proteins such as the NaK2Cl cotransporter. Monoamines and neuropeptide factors have also been shown to play a role. Dopamine, serotonin, melatonin, Atrial Natriuretic Peptide (ANP) and Arginine Vasopressin (AVP) receptors are present on the surface of choroidal epithelium. ANP and AVP decrease CSF secretion [17], as ANP acts on aquaporin I. The variable expression of ANP and AVP receptors according to CSF dynamics appears to be involved in the pathophysiology of hydrocephalus and dementia of the Alzheimer type. Loop diuretics and carbonic anhydrase inhibitors, which act on enzymatic mechanisms to decrease CSF secretion and turnover, could alter the neuronal environment, predisposing to age-related neurodegenerative disorders in the elderly. Cerebrospinal fluid circulation CSF circulation is a dynamic phenomenon and regulation of CSF circulation is responsible for cerebral homeostasis. CSF circulates from the sites of secretion to the sites of absorption according to a unidirectional rostrocaudal flow in ventricular cavities and a multidirectional flow in subarachnoid spaces. CSF flow is pulsatile, corresponding to the systolic pulse wave in choroidal arteries. CSF produced by the choroid plexuses in the lateral ventricles travels through interventricular foramina to the third ventricle, and then the fourth ventricle via the cerebral aqueduct and finally to the subarachnoid spaces via the median aperture (foramen of Magendie) of the fourth ventricle. In the cranial subarachnoid space, CSF circulates rostrally to the villous sites of absorption or caudally to the spinal subarachnoid space. Experimental studies have demonstrated the existence of a communication between CSF spaces and the adventitia of cerebral arteries: red blood cells injected into CSF spaces in the cat pass through the adventitia of cerebral arteries and are then detected in cervical lymph nodes [18]. The CSF, partly absorbed by spinal arachnoid villi, circulates rostrally to the cranial subarachnoid space. CSF flow is generated by the systolic pulse wave and rapid respiratory waves. The subcommissural organ, a differentiation of the ependyma at the rostral extremity of the cerebral aqueduct, appears to play a role in cerebrospinal fluid circulation The subcommissural organ synthesizes SCO-spondin, which has a phylogenetically conserved amino acid sequence [19]. SCO-spondin aggregates to form Reissner fibres, which guide the CSF circulation through the cerebral aqueduct. Rats immunised against Reissner fibres develop hydrocephalus due to stenosis of the cerebral aqueduct [20]. The subcommissural organ disappears early during development in man. An intrauterine abnormality of the subcommissural organ could explain certain forms of congenital hydrocephalus [21]. Cerebrospinal fluid absorption Cerebrospinal fluid is essentially absorbed into the internal jugular system via cranial arachnoid granulations Arachnoid villi are finger-like endothelium-lined protrusions of the arachnoid outer layer through the dura mater in the lumen of venous sinuses [22] (Fig. 1). Obstruction to internal jugular venous drainage is a rare cause of hydrocephalus. The pressure gradient between subarachnoid spaces and the venous sinus necessary to ensure CSF drainage is between 3 and 5 mmHg [23]. The pressure in the superior sagittal sinus remains relatively constant when the CSF pressure is modified experimentally [24]. Spinal arachnoid villi in contact with the epidural venous plexus represent a pathway of CSF absorption especially during effort (Fig. 2). Several different morphological types of arachnoid villi are present in the meningeal sheath of spinal nerve roots: some villi partially cross and others completely cross the dural membranes with various surface areas of exchange according to the degree of plication of the arachnoid layer. In the Green Monkey, arachnoid villi reach the epidural space and penetrate into the wall of veins situated around the spinal ganglion in about 16% of spinal roots [25]. Villous absorption of CSF, either in the brain or in the spine, is a dynamic process which adapts the filtration rate to CSF pressure (Fig. 1). In man, arachnoid villi in lumbosacral nerve roots increase CSF absorption in the upright position in response to gravity, and the absorbed CSF then enters the lymphatic system The role of extra-arachnoid absorption pathways remains poorly elucidated CSF can also be absorbed by cranial and spinal nerve sheaths, the ependyma and extracellular fluid according to pressure gradients. Absorption towards the interstitial compartment occurs via Virchow-Robin perivascular spaces. Cerebrospinal fluid absorption surfaces have been identified on meningeal sheaths CSF absorption surfaces have been identified on meningeal sheaths, particularly the meningeal recesses of spinal and cranial nerve roots, especially the trigeminal nerve and cochlear nerve. The optic nerve, derived from the diencephalon, presents a long extracranial course in its meningeal sheath. CISS MRI sequences of the orbits for assessment of hydrocephalus and benign intracranial hypertension show fluid thickening of the optic nerve sheaths, visualised as a high-intensity ring around the nerve, suggesting participation in CSF absorption when the capacity of the usual circulation-absorption pathways has been exceeded. The cribriform plate of the ethmoid bone has been studied in particular detail Vital stains injected into CSF spaces are subsequently found in the nasal submucosa and cervical lymph nodes [27,28]. This absorption pathway participates in the elimination of proteins and red blood cells from the CSF in cats and rabbits [29,30] and could be involved in the immune defense mechanisms of the brain [31]. The lymphatic circulation would be a preferential pathway of absorption of cerebral interstitial fluid, successively involving perivascular spaces, the arachnoid sheath of olfactory nerve fibres through the cribriform plate, the nasal submucosa and cervical lymph nodes [32]. In sheep, occlusion of the cribriform plate of the ethmoid bone increases the intracranial pressure [33], and lymphatic absorption of CSF increases with increasing intracranial pressure. At normal intracranial pressure, 10% of cervical lymph is derived from CSF, but when intracranial pressure increases from 10 to 70 cm H2O, 80% of cervical lymph is derived from CSF and the cervical lymphatic Figure 3 Cerebrospinal fluid (CSF) ‘‘secretion-circulationabsorption’’ process. CSF is mainly secreted by the choroid plexus and, to a lesser extent, by the interstitial compartment. It circulates rostrocaudally inside the ventricles and drains into the cerebellomedullary cistern (cisterna magna) through the median aperture (foramen of Magendie) of the fourth ventricle. CSF circulates in cranial and spinal subarachnoid spaces. In the cranial subarachnoid space, CSF flows towards arachnoid villi in the wall of venous sinuses from which it is absorbed. Part of the CSF is absorbed by the olfactory mucosa and cranial nerve (optic, trigeminal, facial and vestibulocochlear nerves) sheaths and is drained by the lymphatic system. In the spinal subarachnoid space, the part of the CSF absorbed by the epidural venous plexus and spinal nerve sheaths enters the lymphatic system, while the remaining CSF circulates rostrally towards the cranial subarachnoid space. CSF communicates with interstitial fluid via Virchow-Robin perivascular spaces. flow rate is increased fourfold [34]. Ligation of cervical lymph vessels of the dog induces cerebral oedema [35]. The functional role of this lymphatic pathway in man remains unknown (Fig. 3). These sites of absorptions constitute accessory pathways when the capacities of cranial arachnoid villi are exceeded. They are especially active in neonates, as immature arachnoid villi only become fully functional after the age of 18 months, and in the elderly due to fibrous changes of arachnoid granulations. Recent anatomical studies have focused on the cochlear aqueduct The cochlear aqueduct, situated in the petrous part of the temporal bone, establishes a communication between the subarachnoid space of the posterior cranial fossa and the perilymphatic space of the cochlea. This communication, patent in 93% of cases [36], would explain the impact of intracranial pressure variations on cochlear function, such as tinnitus occurring at high altitude and after ventriculoperitoneal shunting. Raised intracranial pressure modifies the results of certain audiological tests [37]. Cerebrospinal fluid turnover rate CSF is renewed four to five times per 24 hours in young adults. Ageing is characterised by a relative increase of the CSF compartment with respect to the brain parenchyma due to cerebral atrophy and a reduction of CSF turnover to three times a day at the age of 77 years. Catabolites of neurotransmitters and beta-amyloid (A) accumulate in the interstitial compartment, Virchow-Robin perivascular spaces [38,39], choroidal epithelium and ependyma during ageing and also in patients with adult chronic hydrocephalus (ACH) and Alzheimer’s disease (AD). Forty percent of patients with ACH present histological lesions of AD [40]. Decreased CSF A levels have been reported after an internal CSF shunt [41]. Cerebrospinal fluid pressure Cerebrospinal fluid pressure, defined as the intracranial pressure in the prone position, is the result of a dynamic equilibrium between CSF secretion, absorption and resistance to flow. CSF pressure can be measured invasively by a pressure transducer placed in the brain parenchyma or connected to CSF spaces via an external lumbar drain or external ventricular drain. Non-invasive methods essentially consist of interpretation of vascular flow on Doppler ultrasound. A method currently under investigation records the electrophysiological activity of outer hair cells of the cochlea. CSF pressure transmitted via the cochlear aqueduct influences intralabyrinthine pressure and the electrophysiological activity of outer hair cells. Monitoring of outer hair cell activity can therefore be used to monitor intracranial pressure variations [42—44]. Physiological values of cerebrospinal fluid pressure Physiological values of CSF pressure vary according to individuals and study methods between 10 and 15 mmHg in adults and 3 and 4 mmHg in infants. Higher values correspond to intracranial hypertension. CSF pressure varies with the systolic pulse wave, respiratory cycle, abdominal pressure, jugular venous pressure, state of arousal, physical activity and posture. The mechanisms regulating cerebrospinal fluid pressure have not been fully elucidated Cerebrospinal fluid pressure is determined by parenchymal and venous pressures. The cranial content comprises three compartments: parenchymal, venous and CSF. Prior to fontanelle closure, i.e. ‘‘open fontanelles’’, the plasticity of the infant’s skull adapts to pressure increases by an increase in intracranial volume leading to macrocephaly. After closure of the fontanelles, the skull forms a rigid bone chamber. In the presence of an intracranial space-occupying lesion, compensatory reductions of blood volumes (especially venous) and CSF are more active than at the ‘‘open fontanelles’’ state. The capacity of the intracranial contents to adapt to volume changes can be assessed by measuring brain compliance, defined as the volume required to modify intracranial pressure. Brain compliance is measured during a perfusion test, which monitors the increase in intracranial pressure generated by perfusion of saline into the spinal subarachnoid space. Brain compliance is higher in women and varies with age. The volume required to induce a tenfold increase in intracranial pressure is 8 ml in neonates, 20 ml in 2-year-old children and 26 ml in adults. Calculation of brain compliance must take into account the brain volume, which is an average of 335 ml in neonates and 1,250 ml in young adults. CSF pressure is regulated at all levels of CSF hydrodynamics: secretion, circulation, absorption. Increased intraventricular pressure exerts negative feedback on choroidal secretion by decreasing the pressure gradient across the blood-CSF barrier and by decreasing cerebral perfusion pressure. Neuropeptides (ANP and AVP) also appear to be involved. The concentrations of these neuropeptides in CSF and expression of their receptors in the choroidal epithelium increase with increasing CSF pressure and in acute hydrocephalus [45,46]. ANP and AVP decrease choroidal secretion of CSF and induce dilatation of pial arteries, which tends to compensate for the reduction of cerebral perfusion pressure in acute hydrocephalus [47]. Cerebrospinal fluid homeostasis CSF exerts a well-known function: hydromechanical protection of the neuraxis. CSF plays an essential role in homeostasis of cerebral interstitial fluid and the neuronal environment by regulation of the electrolyte balance, circulation of active molecules, and elimination of catabolites. CSF transports the choroidal plexus secretion products to their sites of action. This mode of distribution by CSF circulation modulates the activity of certain regions of the brain by impregnation, while synaptic transmission produces more rapid changes of activities [48]. The wastes of brain metabolism, peroxidation products and glycosylated proteins, accumulate with age-related decreased CSF turnover. Disclosure of interest The authors declare that they have no conflicts of interest concerning this article.










Post a Comment