Introduction
Hyponatremia is the most frequent electrolyte disorder found in cancer patients[1]. It is defined as a serum sodium level (SNa) of less than 135 mmol/L. Hyponatremia is generally classified into mild (130-135 mmol/L), moderate (120-129 mmol/L), and severe (< 120 mmol/L) according to the SNa, and is usually correlated with its symptoms[2], However, the velocity of descent should also be taken into account, as severe, acute hyponatremia is also defined as a drop in sodium levels of more than 10 mmol/L in 48 h. The precise prevalence of hyponatremia in patients with cancer has yet to be determined. The frequency of hyponatremia varies depending on the type of tumor, clinical scenario, and the threshold used for definition of hyponatremia. However, up to 47% of patients in the oncology ward[3] have been found to present hyponatremia. Furthermore, hyponatremia can precede the diagnosis of malignancy, with incidences between 1% and 40%[4].
Historically, hyponatremia has been more frequently associated with small cell lung cancer than with other tumors[5]. However, other publications establish that this hydro-electrolytic alteration can be detected in any cancer patient[3,6]. Direct cancer-induced hyponatremia could be due to ectopic arginine vasopressin secretion, inducing the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH). Furthermore, there is emerging evidence for expression of sodium-transporting proteins in cancer[7].
Hyponatremia can also be attributed to other etiologies that are not related per se to the cancer. What is more, hyponatremia can be induced by cancer-related complications, as well as the anti-cancer treatment itself[8] or the side effects of cancer therapy. These include diarrhea, nausea, vomiting, pain, nephrotoxicity, adrenal insufficiency (due to adrenal metastases), etc.[5].
Hyponatremia can be a potential negative prognostic factor in patients diagnosed with solid tumors or hematological malignancies such as lung cancer, breast cancer, lymphoma, and colorectal cancer[5]. In cirrhosis, hyponatremia is associated with a higher morbidity and mortality. Decompensated cirrhosis in liver cancer patients represents an additional complicating factor[9].
Hyponatremia detected in in-hospital cancer patients is associated with a longer hospital length of stay and an increased risk of mortality[4,6,10-13].
The impact of correction of hyponatremia on patient survival has yet to be ascertained. In some patient series, the correction of hyponatremia correlates with an improvement in quality of life and an improved prognosis[4,14].
The diagnostic approach to hyponatremia in cancer patients
The diagnostic approach in cancer patients should be the same as for any patient with hyponatremia[2,15]. The physical examination is fundamental, since it can establish the volume status of the patient [Table 1]; the clinical history, blood and urine tests are also necessary [Table 2] to determine the etiology [Table 3][16,17].
Physical examination approach to classify hyponatremia regarding volume status[18]
| Physical examination | |
|---|---|
| Orthostatism | Orthostatism can often be found in hypovolemia |
| Manual ocular pressure | Manual ocular pressure can be low in hypovolemia |
| Internal jugular venous pressure | Inspection of internal jugular vein. The maximum height of the pulse of this vein reveals the pressure in the right cardiac atrium: A maximum pulse height below the angle of the sternum in a reclining patient indicates hypovolemia; A height between 1 and 3 cm above the sternal angle indicates euvolemia or hypovolemia with cava vein thrombosis or severe pulmonary hypertension; A height over 4 cm indicates elevated right atrial pressure, as is found in congestive heart failure |
| Edema/increase of liquids in a “third space” | Hypervolemic status usually presents with an increased third space such as edema or ascites |
Basic blood and urine tests for the diagnosis of the cause of hyponatremia
| Blood test (serum) | Urine test | Gasometer |
|---|---|---|
| Protein | ||
| Glucose | ||
| Urea | ||
| Creatinine | Creatinine | |
| Osmolality | Osmolality | |
| Sodium | Sodium | Sodium |
| Potassium | Potassium | |
| Chlorine | Chlorine | |
| Cortisol | ||
| TSH | ||
| T4 |
Approach to diagnosis
| Etiology approach for hyponatremia | |
|---|---|
| The basic hyponatremia approach is based on clinical history, physical examination, full blood test (as described in Table 2), timing of the onset of hyponatremia, symptoms, and type of hyponatremia [Table 4] | |
| Urine sodium: essential for the differential diagnosis of hypovolemic hyponatremia. | With renal sodium loss (Urine sodium > 25 mmol/L): diuretics, bicarbonate intake, primary adrenal insufficiency (Addison’s disease), isolated hypoaldosteronism, and salt wasting syndrome Without sodium renal loss (Urine sodium < 20 mmol/L): gastrointestinal losses (vomiting and diarrhea), burns, hemorrhage, and pancreatitis |
| Urine osmolality: essential for the differential diagnosis of euvolemic hyponatremia | ≤ 100 mOsm/kg: secretion of the ADH is inhibited, polydipsia with or without low solute intake, water intoxication, and administration of hypotonic fluids > 100 mOsm/kg: Secretion of ADH is not inhibited, ACTH deficit, severe hypothyroidism, pain, postsurgical stress, nausea, vomiting, the syndrome of inappropriate antidiuretic hormone secretion, use of thiazides, etc. |
The initial step in laboratory evaluation of hyponatremia, after detecting a sodium level below 135 mmol/L, is to assure that hyponatremia is truly present. High glycemic levels, or mannitol infusion can induce translocational hyponatremia. In fact, total blood or serum sodium levels must always be corrected in patients with hyperglycemia. In patients receiving mannitol infusion, a normal plasma osmolality will rule out true hyponatremia. Pseudohyponatremia, induced by high protein or lipid levels, can be excluded by the determination of total blood sodium by gasometer.
The best and most direct way to ascertain whether the patient presents hypovolemic or euvolemic eunatremia is by neck inspection of the highest point of the internal jugular vein pulse [Table 1][18]. The evolution of serum creatinine together with SNa is also a good parameter. Serum creatinine usually increases when natremia drops in the hypovolemic patient and usually decreases along with hyponatremia in the euvolemic patient[14].
An appropriate intervention depends on determining the timing of hyponatremia onset, the severity of the neurological symptoms, and the volemic classification [Table 4]. This information, together with clinical history and blood and urine tests, is the basis for determining the etiology of hyponatremia [Table 3].
Hyponatremia clinical approach
| Hyponatremia approach | ||
|---|---|---|
| Timing of the onset of hyponatremia | Acute: onset less than 48 h earlier Chronic: onset more than 48 h earlier *Hyponatremia should be considered chronic when timing of onset is unknown | |
| Neurological symptoms | Mild | Impaired capacity for concentration Cognitive deficit Gait disturbances and falls Memory loss Anorexia |
| Moderate | Cramps Drowsiness Headache Nausea Vomiting Asthenia Impaired gait and falls Confusion | |
| Severe | Lethargy Stupor Seizures Coma Respiratory distress Sudden death | |
| Type of hyponatremia: volemic classification | Hypovolemic Euvolemic Hypervolemic | |
Note that a single patient could experience different volemic episodes (e.g., hypovolemic after having been euvolemic) sequentially[19]. That is why clinical examination remains necessary to assure correct management of hyponatremia at any given point of time, and reevaluation of patients is essential.
Hyponatremia in oncology patients is often considered primarily euvolemic, secondary to SIADH[20]. However, some studies have found that hypovolemic or hypervolemic hyponatremia is more prevalent in hospitalized cancer patients[3,6].
The clinician should remember that the diagnosis of SIADH is always a diagnosis of exclusion[21], in a euvolemic patient with a urine osmolality higher than 100 mOSm/kg, in the absence of pain, nausea, diuretics, adrenocorticotropic hormone (ACTH) deficit, diuretic use, or severe hypothyroidism. ACTH deficit is often overlooked, and all patients who are not receiving pharmacological steroid doses should have cortisolemia determined.
Treatment of hyponatremia in cancer patients
Hyponatremia should be treated to both correct clinical symptoms and permit adequate oncological and nutritional therapy. Furthermore, correction of hyponatremia could potentially influence the cancer patient’s quality of life.
A patient who is a candidate for chemotherapy
Severe hyponatremia (Na < 120 mmol/L)
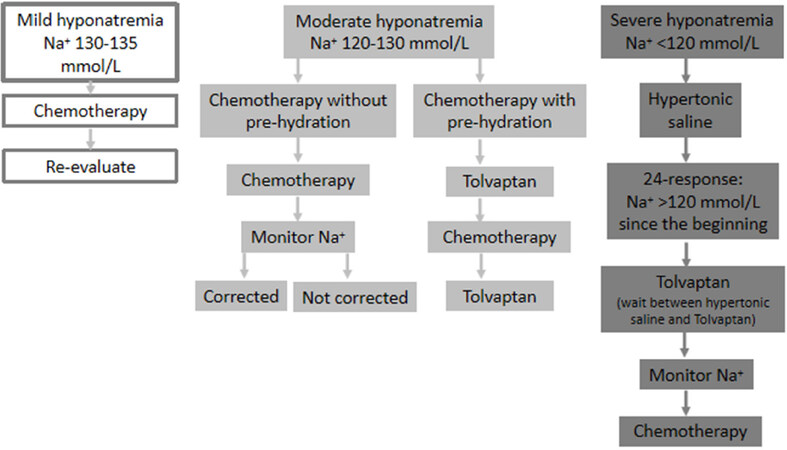
The management of cancer patients is exactly the same as is the case for a non-oncological patient. Hypertonic saline solution (3% sodium chloride) should be administered in i.v. infusion or in bolus therapy, regardless of the type or etiology of hyponatremia. The rate of correction will vary if hyponatremia is chronic or acute. In acute hyponatremia (< 48 h), there are no established limits for correction of hyponatremia. In chronic hyponatremia (> 48 h) or when the timing of the onset of hyponatremia is unknown, the goal of correction in the first 24 h should be a SNa rise of 4-6 mmol/L, reached during the first 6 h of treatment, to reduce cerebral edema [Figure 1].
Figure 1. When the patient is a candidate for chemotherapy (modified from Escobar et al.[29])
Additional treatments for hyponatremia must be avoided during the first 24 h of correction, except for associated furosemide in patients with heart failure or the addition of potassium chloride in patients with initial hypokalemia[2,14][Table 5][22].
The 24- and 48-h goals for correction of chronic hyponatremia (adapted from[22])
| ODS risk | Minimum 24 h SNa rise (mmol/L) | 24 h Goal (mmol/L) | Maximum 24 h SNa rise (mmol/L) | 48 h Goal (mmol/L) | Maximum 48 h SNa rise (mmol/L) |
|---|---|---|---|---|---|
| Low | 4-8 | 6-8 | 10-12 | 6-8 | 18 |
| High | 4-6 | 6 | 8 | 4-6 | 8/day |
Hypercorrection of SNa should be avoided in patients with chronic hyponatremia (> 48 h from onset) or when the timing of the onset of hyponatremia is unknown. Patients presenting risk factors for the Osmotic Demyelination Syndrome (ODS) (hypokalemia, malnutrition, liver failure, and alcoholism) should not present 24-h SNa rises above 8 mmol/L during the first or second 24 h of therapy. Therefore, following Hypertonic saline therapy, SNa should be monitored every 6-8 h. If SNa re-descends, hypertonic saline can be administered anew. If SNa levels have reached the desired goal hours before 24 h have elapsed since the start of treatment, desmopressin (DDAVP) can be associated at doses of 1-2 μg every 6-8 h until the 24 h have elapsed, to prevent SNa levels from continuing to increase. Diuresis should be monitored and DDAVP administered in the case of polyuria.
A marked exception to the use of hypertonic saline is the suspicion of an adrenal crisis. In this case, established protocols for treatment should be applied, with i.v. hypertonic saline only used if the patient’s SNa fails to rise adequately[2,19].
Mild/moderate hypovolemic hyponatremia
Management will depend on the cause of hyponatremia. Diarrhea and vomiting are frequent side effects of chemotherapy. Antiemetics and adequate hydration and salt intake can be enough to correct non-severe hypovolemic hyponatremia, although i.v. isotonic saline can be required.
If it is mild hyponatremia, the patient can proceed with chemotherapy and control serum sodium levels in outpatient clinic.
Mild/moderate hypervolemic or euvolemic hyponatremia
In patients with a diagnosis of SIADH, fluid restriction can be attempted if patients are not candidates for surgery, hyperhydration, or nutritional supplements or support. Furthermore, patients at nutritional risk could limit protein intake when reducing fluids, as the latter are usually needed to eat solids. Fluid restriction refers to all administered liquids, including i.v. medication and semisolids administered in the diet.
Fluid restriction should not be attempted if the patient’s biochemistry indicates their kidneys are unable to eliminate free water. To ascertain response, the Furst formula can be applied.
The first approach is fluid restriction and increased salt intake in diet, applying the Furst formula[23]: (Urine sodium + Urine K)/Serum sodium
The result of this formula will predict whether fluid restriction will be effective for the treatment of hypervolemic or euvolemic hyponatremia, and the threshold of the liquid restriction: (1) < 0.5: fluid restriction of < 1000 cc/day; (2) 0.5-1: fluid restriction of < 500 cc/day; (3) > 1: fluid restriction ineffective.
In cancer patients with a high prevalence of and risk for malnutrition and the frequent need for dilution of chemotherapy, fluid restriction is often not viable[14,19].
Evidence in favor of the use of fluid restriction in patients with hypervolemic hyponatremia is scant[24]. However, its use has been incorporated into therapeutic algorithms of congestive heart failure. If there is no response to fluid restriction, Tolvaptan could be considered[25].
Patients who are not candidates for chemotherapy
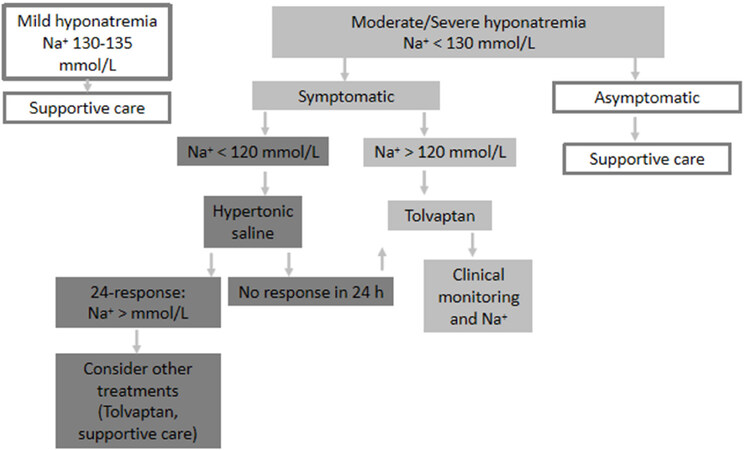
Supportive treatment and treatment of hyponatremia should be prioritized according to the severity of the symptoms, rather than the level of hyponatremia per se[Figure 2].
Figure 2. When the patient is not a candidate for chemotherapy (modified from Escobar et al.[29])
Hyponatremia due to SIADH in cancer patients
Tolvaptan is approved for the treatment of SIADH-induced hyponatremia in adult patients[26-28].
The Spanish Medical Oncology Society (SEOM) has developed an algorithm which could be useful for the management of hyponatremia secondary to SIADH in cancer patients[29] based on the prior algorithm developed in Spain for hyponatremia patients[30].
According to the SEOM algorithm, management will depend on the two scenarios mentioned above: the patient is a candidate vs. a non-candidate for chemotherapy.
When the patient is a candidate for chemotherapy [Figure 1]
Mild hyponatremia (130-135 mmol/L): proceed with chemotherapy. Re-evaluate during the next cycle. Note that there are discrepancies in this point, as Tolvaptan would prevent the exacerbation of hyponatremia and the development of severe hyponatremia following the first cycle of chemotherapy[
19 ];Moderate hyponatremia (120-130 mmol/L): consider Tolvaptan if chemotherapy requires pre-hydration, hyponatremia is progressively worsening, or hyponatremia is symptomatic. As mentioned above for mild hyponatremia, Tolvaptan could prevent worsening hyponatremia;
Severe hyponatremia (< 120 mmol/L): use the same as treatment as for severe hyponatremia. Once 24-48 h have elapsed following therapy with hypertonic saline solution, Tolvaptan could be started.
When SIADH-induced hyponatremia is caused by the anti-cancer treatment itself (for example, vincristine)[8], a modification of cancer therapy should be considered. When a change in medication is not feasible, SIADH in these cases should be treated with Tolvaptan.
When the patient is not a candidate for chemotherapy [Figure 2]
As mentioned above, supportive treatment and treatment of hyponatremia should be prioritized according to the severity of the symptoms, rather than the level of hyponatremia per se:
Mild hyponatremia (130-135 mmol/L): focus on supportive care management;
Very symptomatic moderate/severe hyponatremia (< 130 mmol/L): consider Tolvaptan. If < 120 mmol/L, treat the acute phase with hypertonic sodium solution and, once stabilized, consider Tolvaptan;
Mild symptomatic moderate/severe hyponatremia (< 130 mmol/L): focus on supportive care management.
إرسال تعليق